IDefine Impact Report 2022

With the end of 2022, we mark the second year of IDefine’s existence. We are moving with an incredible sense of urgency, moving our way towards a targeted treatment to combat the challenges put upon our loved ones with Kleefstra Syndrome and those caring for them. In just two years, we have established ourselves as a leading organization in the rare disease community with our commitment to our three pillars of focus: Building the Kleefstra Syndrome community, Developing Coordinated Clinical Care for patients with Kleefstra Syndrome, and Propelling Research towards a therapeutic intervention for Kleefstra Syndrome. In 2022, we made significant strides across all three pillars.
As we have said before, we yearn for the day of “no more nevers.” We pray for the day when our loved ones aren’t suffering from Kleefstra Syndrome. Tragically, our community mourned the loss of six beautiful souls with Kleefstra Syndrome in 2022 alone. We must intervene and build a brighter future for our loved ones.
We have a small but mighty team working collectively to further our mission and create opportunities, and we are grateful to our donors who enable this work and fuel our hope.
2022 Foundation Highlights
- Selected to attend the Ultragenyx Rare Entrepreneur Bootcamp with only 20 other rare disease foundations.
- Hosted our first ever in-person North America Kleefstra Syndrome Family Conference with parents, families, clinicians, scientists, and special needs experts from all over the country (and world thanks to Dr. Vermeulen!)
- Hosted our 2nd annual International Kleefstra Syndrome Family + Scientific Conference (virtual) with a consortium of clinicians and scientists from all over the world who are furthering our knowledge and understanding as we move towards better care and a cure.
- Invited to join CombinedBrain, a nonprofit consortium brings together 30 patient advocacy groups to speed the path to clinical treatments for people with severe rare genetic, nonverbal neurodevelopmental disorders by pooling efforts, studies and data.
- Attended the annual Global Genes RARE Patient Advocacy Summit, one of the world’s largest gatherings of rare disease patients, caregivers, advocates, healthcare professionals, researchers, and partners.
2022 Research Highlights (see details below)
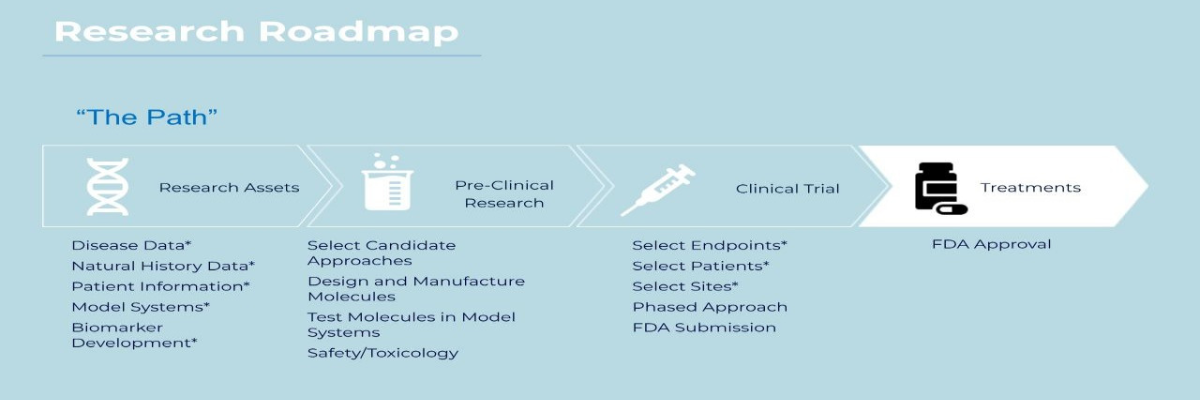
- Drafted a Research Roadmap to lay out near term and future areas of focus that lead us closer to clinical trials. Our next big step falls under Preclinical Research and includes selecting an approach to a treatment.
- Formed Biomarkers and Endpoints Consortium for Kleefstra Syndrome (BECK).
- Launched an International Study on Speech + Language for Kleefstra Syndrome with Professor Angela Morgan and PhD student Lottie Morison that seeks to further our understanding of speech and language abilities in Kleefstra Syndrome.
- Launched a project to develop iPSC’s – stem cell line models that have been created with Kleefstra Syndrome – that can be used to test potential therapeutics.
- Launched Kleefstra Syndrome sleep study, led by researcher Lara van Renssen, and with the support of Dr. Tjitske Kleefstra.
- Co-sponsored CANDID, a collaboration between patient advocacy groups, scientists, clinicians, and research organizations that is focused on addressing gastrointestinal dysfunction in patients with neurodevelopmental disorders like autism.
Kleefstra Syndrome Community and Patient Data
- Launched the Kleefstra Syndrome Worldwide Map and Census Project to gather accurate data on how many patients currently exist.
- Enrolled more than 100 individuals with Kleefstra Syndrome into our RARE-X data collection platform in order to accelerate research and the development of new drugs, devices, or other therapies.
- Enrolled more than 100 people with Kleefstra Syndrome into AllStripes, a platform that centralizes medical records for families while helping guide treatment research.
2023 Goal: Drive the science to clinical drug development
- Support, encourage, and empower our community through their rare disease journey and towards our mission.
- Conclude and present to the community the Kleefstra Syndrome Clinical Guideline project.
- Fund two discovery phase projects focused on either ASO’s, gene therapy, or small molecules.
- Fund further understanding of Kleefstra Syndrome symptoms through studies, such as a disease concept model.
- Continue to expand our community through our Worldwide Map and to further develop our understanding through our data collection initiatives.
- Raise financial reserves to prepare for clinical drug development.
- Connect and partner with biopharma to include Kleefstra Syndrome in its pipelines.
- Continue to host family and scientific conferences.
THANK YOU
We aim to be relentless in our pursuits to build a brighter future for all of those impacted by Kleefstra Syndrome. We fully recognize that our effort is one that is built on, by, and for our community. We are incredibly grateful to every person who is on this journey with us. On behalf of the IDefine team and our international community, thank you for your support. Looking forward!


